|
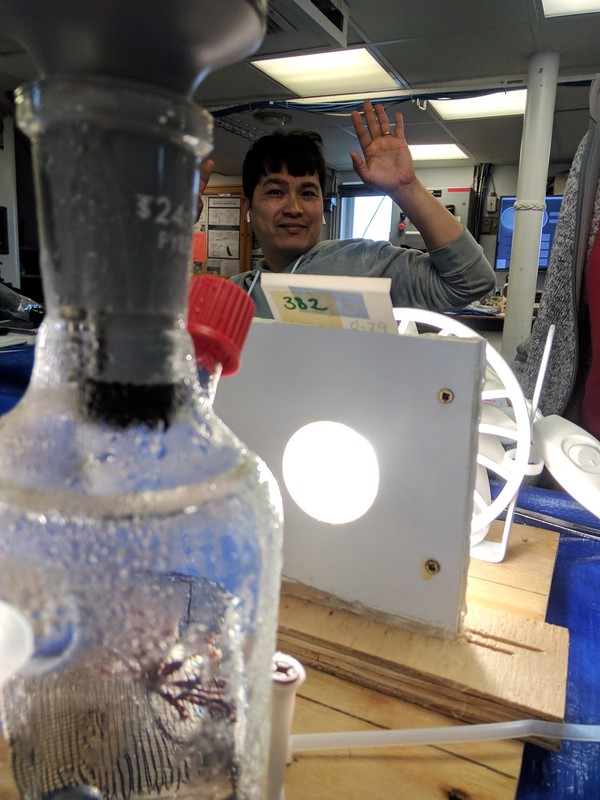
As a senior at San Diego State University, and volunteer with the Edwards lab, I was asked to partner on a project to help identify the invertebrate composition in cores taken in rhodolith and sand (control) samples collected from Catalina Island. Master’s candidate and primary coordinator, Billie Beckley, took the time to introduce me to the project in the early spring of 2018. This project was made possible through the California Sea Grant and Principle Investigator (PI) partnership with Dr. Diana Stellar at Moss Landing Marine Lab. The primary research objective is to ascertain the impacts mooring lines might have on rhodolith beds around Catalina. The data will be used as a baseline for rhodolith ecosystems worldwide in order to study and preserve the complex habitats they provide for other animals. The initial cores were collected in July of 2018. They were photographed underwater immediately following extraction and preserved in a -80°C freezer until thawed out to be sorted using a microscopic. The pictures were used in identifying anoxic sediment content along with rhodolith composition through the utilization of ImageJ® software. Below is a sample picture that was used in this process: Although scrutinizing samples multiple hours a week under a microscope may sound monotonous and boring, it was far from it! Every day had its own set of highlights and interesting discoveries. With a sense of curiosity, new organisms were found or different variations of the same order of invertebrates were observed. For example, an interesting mite-like organism popped up a couple times in some of the cores. Below are pictures of both sides of the specimen: Another notable moment in my sorting process was observing different morphological characteristics between Tanaids found in nearby sand beds verses those in the rhodolith beds. In the sand, Tanaids were a whiteish, opaque color whereas the Tanaids that were living in the rhodolith beds were opaque with darker colored spots on their carapaces. Although this is not explicitly part of the primary research question, it would be interesting to know what factors allow for phenotypic variation between the two taxa. From January 8-18, 2019, members from both affiliated labs will go back to Catalina to gather more core samples and bring them back to SDSU to sort and analyze. Photosynthetic measurements through the use of Photosynthetic Irradiance (PI) curves will be used the same day they are collected so that we have a better understanding of the overall productivity in these ecosystems. So stay tuned for more updates from the field and the lab! Natalie Goetz San Diego State University B.S. Marine Biology, 2019 California Seagrant Funding R/HCE-04  One of the disturbances in the mooring field is the blocked sunlight from the boats themselves One of the disturbances in the mooring field is the blocked sunlight from the boats themselves July 18th 2018 Wrigley Institute for Environmental Studies We’re just now wrapping up our first of four expeditions to Catalina Island’s rhodolith beds, and boy do we have a lot of data to process! In total, we’ve sampled five beds for fish, invertebrates, benthic cover (including cores) and set up 30 CBITs! We’ve talked about what we’re doing out here, and how it’s done, but the story won’t be complete until the data are analyzed. As ecologists, we try to balance field work with “office” work. While we’re often daunted by the enormity of trying to sample an entire ecosystem, the magic really happens when we analyze our data. But more on that later! Clearly, we’ve spent a lot of time underwater on this trip, maximizing our sampling effort and taking advantage of the incredible conditions. At first glance, the rhodolith beds look pretty plain and simple. But as we’ve shown, they’re a lot more dynamic than they appear. If you spend enough time in the ocean you’ll see some amazing organisms, and the rhodolith beds are no exception. From competitive macroalgae to charismatic vertebrates, check out some of the best biota the beds have to offer! California Seagrant Funding R/HCE-04  A core sample, one of eight, taken from a rhodolith bed. We see healthy rhodoliths on top of anoxic sand. Image courtesy of D. Steller A core sample, one of eight, taken from a rhodolith bed. We see healthy rhodoliths on top of anoxic sand. Image courtesy of D. Steller “We speak for the rhodoliths”. But first we need to learn their language. Of the 10 scientists on this expedition, only three of us (project PIs Dr.’s Matthew Edwards and Diana Steller, and PhD candidate Scott Gabara) have worked in rhodolith beds before. That means that the rest of us have a lot of learning to do. And quickly! After a few “shake-out” days, we finally have our sampling protocol dialed in: subtidal surveys, collapsible benthic incubation tents (CBITs), and lab based incubations. Dr. Steller is leading the survey dives, while Dr. Edwards and the Edwards Lab is in charge of the CBITs. We’re incredibly fortunate to have Dr. Ju-Hyoung (who joined us in the Aleutians) working tirelessly in the lab running seemingly endless incubations. Most of the divers rotate through the survey dives; it takes six or so divers at least three dives to effectively survey a rhodolith bed. Each dive team, consisting of at least two divers, is responsible for a piece of the survey puzzle. For example, a dive team might be running fish transects, which consist of four surveys for fish inside and outside of the rhodolith bed (i.e. over an adjacent sandy bottom). During this time, another team is conducting four transects for invertebrate cover, uniform point contacts (UPC), and quadrats for percent cover. We also take eight cores inside the rhodolith bed. These cores help us understand the size frequency distribution of individual rhodoliths, and what the sandy bottom looks like under the rhodoliths. That’s a lot of work just to understand who is in and around a rhodolith bed! We’re coupling these surveys with our CBIT incubations. Just the like larger “chambers” we used in the Aleutians, these “chamberitos” allow us to estimate in situ productivity and respiration. That is, what exactly goes on in a community dictated by these coralline algae. The rhodoliths themselves make up the community we’re studying, but the individuals produce and consume oxygen just like any other photosynthetic being. We want to know what net productivity (photosynthesis minus respiration) looks during over a daily cycle. Our CBITs are secured to the benthos via heavy chain and contain a fixed volume of sea water. Inside this CBIT the community goes on producing and consuming oxygen without being disturbed by the experiment. The flexible design allows for the transfer of water motion, and the polycarbonate panels allow undistorted sunlight in without trapping it like a green house. We leave an oxygen and temperature sensor along with a sunlight sensor inside the CBIT to record daily changes. It’s all well and good to study the components of the community, but our metrics of community composition and Net Community Productivity (NCP) aren’t complete without a detailed understanding of individual contributions to NCP. Enter our productivity expert, Ju-Hyoung. He flew all the way from South Korea to help us understand what individual organisms, including rhodoliths, are capable of from a physiological perspective. All day long Ju-Hyoung is measuring how much oxygen heterotrophs (organisms that only consume oxygen, like humans) consume, and how much oxygen autotrophs (organisms like plants) produce and consume in a rhodolith bed. Our days are long. But the water is warm, the visibility is great and everyone is excited to continue exploring! California Seagrant Funding R/HCE-04  The Isthmus Mooring field is a crowded place! The Isthmus Mooring field is a crowded place! This is Pike aka Baron von Urchin coming at you from Catalina Island. Getting ready for field work is no easy feat, especially when you kick of a 10-day expedition at 4 o’clock in the morning. But that’s just what the Edwards Lab, accompanied by Dr. Ju-Hyoung Kim from Korea, did in the pre-dawn humidity on July 10th. After doing our final checks to make sure all of our equipment was properly stowed, we piled into the lab’s suburban, 21ft dive boat in tow, and headed from San Diego to San Pedro. At around 6:30am we met up with Dr. Diana Steller and another graduate student from Moss Landing Marine Labs (MLML) at the Southern California Marine Institute. There we loaded our gear onto USC’s Miss Christy, the small mainland-Catalina ferry, and prepared to cross the water to Catalina Island. During the summers of 2016 and 2017 the Edwards Lab, along with the Konar Lab from the University of Alaska Fairbanks, studied productivity in kelp forests along the Aleutian Archipelago. This time we’re doing a similar study in another kind of algal-dominated community: rhodolith beds. Rhodoliths (rhodo meaning “rose”, but in this case “red algae” and lith meaning “rock”) are rock-like “balls” of coralline red algae that can form massive beds on soft sediments in clear water. These little “tumble weeds of the sea”, as PhD candidate Scott Gabara calls them, can support a rich diversity of marine organisms in nearshore environments (Gabara 2018). While kelp forests dominate temperate waters on rocky reefs, rhodolith beds thrive in sandy habitats, especially in protected sandy coves. Which, as it turns out, is an excellent place to establish a mooring field for boats. These moorings, where boats tie up overnight, are anchored to the ocean floor (known as the benthos) by heavy chains and massive concrete blocks. Scientists have known for a long time that these mooring chains can crush rhodoliths, turning the once vibrant beds into coralline rubble patches. Scott, who did his Masters on rhodolith beds with Dr. Steller, aka Di, at MLML, showed that benthic diversity is significantly greater inside the beds than outside. Which brings us to the purpose of this first of four expeditions to Catalina Island. In conjunction with Dr. Steller and several graduate students from MLML, the Edwards Lab plans on repeating our Collapsible Benthic Incubation Tent (CBIT) experiments inside and outside of rhodolith beds so we can finally understand just how productive these rolling red communities are. Di will be leading SCUBA surveys for diversity, and overall rhodolith community structure, while Ju-Hyoung will be conducting incubation experiments in the lab on individual rhodoliths, and the marine organisms that make their home among the algae. Our base of operations is USC’s Wrigley Institute for Environmental Studies, located adjacent to Two Harbors on the west end of Catalina. We’ve got two boats, the 21ft Stillwater Cove, and the 12ft inflatable Kenner (last seen in the Aleutians), 10 scientists and 10 days to learn as much as we can about rhodolith beds, the communities they support, and the consequences of their loss. Be sure to follow along for more rhodolith-related action! California Seagrant Funding R/HCE-04  Topside: the crew from OpenROV and Dr. Edwards (red beanie) prepare to test a Trident Topside: the crew from OpenROV and Dr. Edwards (red beanie) prepare to test a Trident It’s been a longtime coming, but we’re incredibly excited to report on our debut dive day with ARREE. In collaboration with OpenROV, our team was able to conduct dive operations concurrent with a Trident test flight in Stillwater Cove, Carmel, California. Stillwater Cove (SWC) sits within the Monterey Bay National Marine Sanctuary is one of the best places to study kelp forest ecology. In conjunction with the Edwards Lab’s focus on kelp forests community dynamics, we have been exploring the formation of sea urchin barrens, and the differences between these two systems. In order to aid in our understanding of the differences in processes between a community dominated by photosynthetic kelps, and those dominated by voraciously herbivorous sea urchins, we have been deploying experiments from Baja California to the Aleutian Archipelago in Alaska. Our experimental set up in SWC provided the perfect opportunity to collaborate with OpenROV and their Trident Drones.  Fair weather greeted us in the usually gloomy Monterey Bay. BEERPIG Billie Beckley warms up after a chilly dive Fair weather greeted us in the usually gloomy Monterey Bay. BEERPIG Billie Beckley warms up after a chilly dive We were thrilled to have OpenROV employees Zack, Mike, and Nicole join us as we explored the vibrant kelp forests and adjacent sea urchin barren grounds in SWC. With the help of our newest lab mate, ARREE the Trident, we will continue exploring and document the changes kelp forest communities are experiencing in an age of unprecedented social and ecological change. Join us as we continue to explore autotrophic communities (those dominated by photosynthetic organisms likes sea grasses and algae) along the Eastern Pacific. We’ll be taking ARREE to Catalina Island this summer to explore fascinating algal communities called Rhodolith beds, and the human impacts to this fragile yet vital ecosystem. Cheers, Baron von Urchin (aka Pike)  The biodiversity of the Aleutians is evident across all of the rocky reef habitats, even in the urchin barren grounds. Photo cred: M. Good The biodiversity of the Aleutians is evident across all of the rocky reef habitats, even in the urchin barren grounds. Photo cred: M. Good Hi all, this is Dr. Edwards, project PI Our second cruise to the Aleutian Islands has come to a close. On July 23, we finished our last set of dives at Yunaska. Yesterday’s rocking and rolling swell was non-existent; the winds had died down completely, and the sun had come out. Yes... the sun. It had been almost two weeks since we saw a clear view of that yellow star that warms us. We went in and hauled our chambers and the hundreds of pounds of chain to the boat and picked up the sensors. The water was so calm, you could set things down and they did not move. The thick kelp that only the day before had snagged on every edge of our tanks, BCs and regulators, now cooperated as if we were in harmony. You could move through it with ease, but only if you respected it...danced with it. If you took it for granted, tried to change the dance, it would still snag and pull on you, slowing every movement. But with respect, it was like gliding through a photograph. The kelp was so thick and full of life. There was an understory of kelp so dense you could not see the bottom. There were sponges that covered square meters of rock bottom. Red, yellow, white. There were large nudibranchs plowing through the sponges and eating their fill. There were snails, and bottom fishes of every color you could imagine. Visibility was so good that the only thing that limited your vision was the scenery in front of you. Though you could if you wanted to, it was hard to pass up an infinite number of scenes in front of you that you almost forgot there was a horizon beyond. It made me completely forget the 41 degree water and the holes in my gloves’ fingertips that appeared after two-weeks of deploying benthic chambers, hauling chain, and collecting urchins. Afterwards we had a day of cleaning gear, packing research equipment, and preparing to disembark from the Oceanus. This is my last shipboard blog for Brenda's and my NSF 2016 and 2017 cruises. In reflection, I look back at what we accomplished. In the past two years, we dove at 15 of the Aleutian Islands (from west to east: Attu, Nizki, Allaid, Shemya, Kiska, Amchitka, Ogliuga, Tanaga, Adak, Atka, Yunaska, Chuginadak, Umnak, Anangula, and Unalaska). My students (Scotty, Tristin, Pike, Sadie and Genoa) and my collaborator Ju-Hyoung pulled off a Herculean amount of work. We deployed nine benthic respiration chambers at each of 11 of the islands; three each in the kelp beds, urchin barren grounds, and in the transition zones between the two. This equated to 99 chamber deployments in total. Each chamber was held down with about 40 pounds of chain, so you can do the math; we deployed and retrieved about 3960 pounds of chain from small inflatables. This, plus the chambers and the sensors…again…Herculean. Together, we did about 250 dives on each of the cruises (about 500 dives total on the project). Conditions ranged from Atka last year where we endured smashing waves and blowing winds in our sites to the calm clear beauty of Yunaska. Water temperatures ranged from 41 degrees to a balmy 46. My students (the SDSU BEERPIGS) more than conquered these tasks and I am grateful and impressed by their abilities, energy, and attitudes. A job well done. On the ship, Ju-Hyoung and Sadie conducted controlled measurements of invertebrate respiration and algal photosynthesis. Generating photosynthesis versus irradiance (P vs I) measurements requires careful and methodical work. The instruments we use are sensitive to even a single degree of change in water temperature, and to differences in light intensity so small that the human eye cannot make them out. Each measurement for each species requires up to 90 minutes to fully evaluate the photosynthetic responses of the algae to changes in irradiance. Between the two of them, they conducted a total of 150 P vs I measurements across 26 species. In total, this equates to about 225 hours of measurement time (and that’s in just four weeks of ship time). Further, they conducted respiration measurements on dozens of species of invertebrates, and evaluated the relationship between urchin respiration and urchin size across nearly 20 size classes. They also examined the carbon uptake kinetics of 10 species of algae, and helped parameterize our field irradiance measurements with light casts. Another job well done.  Scotty took on a behemoth of a project. We're all looking forward to his results. Photo cred: P. Spector Scotty took on a behemoth of a project. We're all looking forward to his results. Photo cred: P. Spector On top of deploying and retrieving chambers, Scotty collected tissue samples from about 400 invertebrates, and 101 water samples that were filtered for phytoplankton. Over the next year, he will analyze these for their isotopic signatures to better understand how food webs and trophic energy flow has changed with the widespread kelp loss. Together with the benthic respiration chamber data I see my next several months of work analyzing and interpreting the results. And then writing up the papers. Not as fun as being underwater, but just as important and rewarding. The crew of the Oceanus was amazing. From the captain on down, everyone was friendly and incredibly helpful. Our skiff drivers navigated the dense kelp beds, and helped haul heavy chain and chambers into the boats. They were both our drivers and our surface support. The food was awesome, from fresh-caught baked halibut to hot out-of-the-oven cookies waiting for us when we returned from our dives, worn out and cold. We could not have asked for a better crew or ship. I thank the National Science Foundation for providing both the ship and the funding for this research.  A job well done! Both labs pose at the entrance to the collaborative exhibit at the Museum of the Aleutians in Dutch Harbor A job well done! Both labs pose at the entrance to the collaborative exhibit at the Museum of the Aleutians in Dutch Harbor So, as I close this blog, I think about the next time Brenda and I will bring our labs here. It has been almost 25 years since I first came here to live for a few months on Shemya; this place is as beautiful and new as the day I first stepped on that tiny island (though I am a little greyer and maybe a step slower). The ecosystem offers an endless avenue of research questions and our research (as most research does) has resulted in as many new questions as it provided answers. So, I think of the last dive at Yunaska, sitting mid-water and looking at the dense kelp teaming with life. I think of the urchin barrens, which at one time looked to me to be devoid of anything other than urchins, but now have revealed themselves to be diverse and full of their own life. What will happen next? How long will the urchin barrens persist? What might cause them to switch back to kelp beds? New predators? Disease? Some as-of-yet unknown factor? The questions remain. I think about the last dive, and I believe the Aleutians are calling me back. Sincerely, Dr. Matthew Edwards Project PI Kelp loss, urchin barrens, and food web comparisonsHey everyone, Scott Gabara here to describe part of my dissertation working on creating food webs of nearshore and offshore subtidal communities across the Aleutians Islands. In the marine environment, kelps can create the understory and canopy that comprise most of the biomass of kelp forests, providing structure and food resources for their associated communities and can even deliver material to adjacent areas supporting consumers many kilometers away! This is very similar to the plants, bushes, and trees that comprise forests on land (and can deliver leaf litter to adjacent areas). With the loss of sea otters and subsequent formation of urchin barrens across many locations of the Aleutian Island archipelago, kelp is lost and organisms will either not be able to deal with the loss of food that kelps provided, or they may change their diet to another source of food locally, or rely on food from other locations that drift in. At some locations urchin barrens were formed long ago, but most formed during the late 1990s- early 2000s, giving us communities that lost kelp long ago, many that have lost kelp for about 20 years, and some locations that have had kelp continuously. I wanted to better understand the long term impacts of kelp loss on the trophic ecology of these kelp forest communities that still have kelp or have lost kelp over different amounts of time. To do this I will create and compare food webs using stable isotopes of the dominant community members (producers and consumers) among urchin barrens, kelp forests, and offshore communities at islands across the Aleutian archipelago.
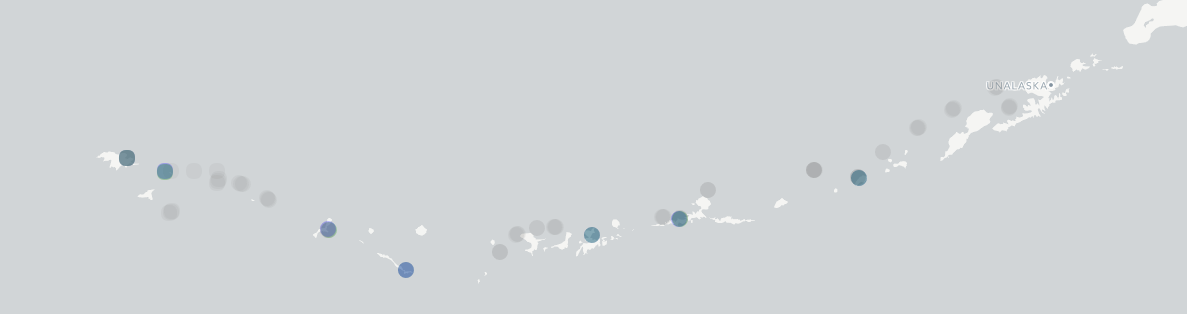
Stable isotopes of carbon and nitrogen are stored in tissues and help us to understand how energy flows through food webs. Stable isotopes act as a hidden tracer that can reveal both where energy is coming from, and the trophic level of a consumer. For example, we can sample the fingernails or hair of people, and using carbon stable isotope values, be able to tell differences of diets based more on rice versus those based more on corn because of the differences in the way rice and corn take carbon up during photosynthesis. We can also identify differences in the diet of people that are vegan, vegetarian, or those that eat more meat, because of differences in the trophic level of the foods that they eat. All of this information is stored in the nitrogen stable isotopes of the food we eat and is then left as a signature in our fingernails and hair. Food webs help us to understand the connections among organisms and reveal the potential impacts of removing certain food resources (primary producers) that comprise the base of the food web or the impacts of removing the food web members (consumers). Potential food web resources for kelp forest and urchin barren associated organisms are things in the water such as phytoplankton (that live in the water column) or pieces of algae that break off while algae grow or get ripped off the bottom and drift around, multiple attached algae such as the lower lying green and red algae in kelp forests, and the larger subcanopy and canopy forming brown algae. To estimate a water column isotopic signature, water was filtered from urchin barrens, kelp forests, and offshore. Filtering water for phytoplankton or algae particulates is no easy task. Water is collected and then needs to either be pushed or pulled through a filter to concentrate the material from the water. To do this I modeled a water filtration setup after the Rohwer lab at SDSU/Smith lab at Scripps developed by Mark Hatay at SDSU. Clear PVC was cut to fit 5 Liters of water, 4 inch adjustable plumbing plugs were used to seal the ends, and tapped plastic valved fittings onto the plugs were used to create a tube that could collect water and be pressurized. A propane pressure regulator and a SCUBA tank was used to create pressure that would push the water through the clear collection tube and then through a glass fiber filter in a holder, concentrating the material from the water on the filter, to be dried and later run for stable isotope analysis. Locations of nearshore and offshore (grey), kelp forest (green), and urchin barren (blue) water samples.
To estimate algal isotopic signatures, tissues of algae that were living in urchin barrens and kelp forests were collected. To estimate the isotopic signature of nearshore and offshore consumers, invertebrates were collected from surveys or offshore trawls done in collaboration with the Konar lab at University of Alaska Fairbanks. To estimate fish isotopic signatures, pole spears were used to catch fish that were in urchin barrens and kelp forests by divers and by using the trawl to get fish that were living in the deeper offshore areas.
Tissues were prepped and dried on the R/V Oceanus and will be run for carbon and nitrogen stable isotope analysis. The ratio of the heavier to lighter stable isotopes of carbon and nitrogen will help us trace how important local and/or drifting kelp is for organisms in urchins barrens, kelp forests, and offshore areas. By creating food webs of these different communities we may have a better understanding of why certain organisms can survive the transition from kelp forests to urchins barrens and if and how kelp loss in the nearshore affects offshore food webs.
 Benthic respiration chamber deployed in a transition zone. Photo by Scott Gabara Benthic respiration chamber deployed in a transition zone. Photo by Scott Gabara Sadie here with a brief expedition update and a look inside shipboard lab operations! The next island along our route was Agattu, but inclement weather forced us to head East sooner than expected. After a full travel day, we arrived at beautiful Kiska Island this morning. As usual, the Edwards Lab dive team deployed chambers in three different habitat types (kelp, transition, and urchin barren). A quick refresher on what our lab is doing up here in the Aleutians: we’re comparing ecosystem productivity between these three habitats and among different islands in an east-west gradient along the island chain. For this large-scale project, we use clear, flexible chambers to isolate 0.57m^2 patches of the seafloor for 24-hour periods, meanwhile collecting changing oxygen concentration, temperature, and light data within these chambers. The organisms in nearshore benthic habitats all use or produce oxygen during photosynthesis or respiration, so we use oxygen concentration to assess the activities of algae and animals within the chambers. These data help us estimate productivity from the community as a whole; however, we want to go deeper into the story! Algae and invertebrate animals from inside the chambers are collected and brought back to the ship and further analyzed to help us understand how individual members of the community are contributing to our large-scale production estimates.  That’s where Dr. Ju-Hyoung Kim and I come in. We’re using small-scale methods at the individual level to add details to the large-scale benthic chambers that give us community-level data. In the shipboard laboratory, we have set up small glass incubation chambers that hold one organism at a time. By controlling temperature and light, we can measure the change in oxygen concentration via photosynthesis (algae) and respiration (animals). Coupled with biomass data from the chambers, we hope tease apart how each species contributes to the larger productivity estimate within the benthic chambers that the dive team deploys in the field. These experiments in the field and in the laboratory allow our group of scientists to come together and work toward a common goal, which is to understand more about the valuable ecosystems of the Aleutian Islands!  Usually our common goal is research-related, but sometimes we get together and make algae art! Featured here in an herbarium pressing: Neoptilota asplenoides, Kallymeniopsis sp., Odonthalia setacea, Neinburgia prolifera, Callophyllis sp., and more. Catch you at the next island!
--SS  Before each deployment the Edwards Lab prepares the sensors by attaching them to purpose-made stands Before each deployment the Edwards Lab prepares the sensors by attaching them to purpose-made stands Nizki Island 7/14/2017 Hey there, this is Pike coming at you again from the Semichi Islands. Another day, another deployment. We’ve been in the Aleutians aboard the r/v Oceanus for just over a week now, diving in frigid water almost every day now. After trawling on Attu, we began our steam eastward; in the pre-dawn gloom we “dropped hook” in a small island cluster known as the Semichis. As we stumbled out of our bunks and towards the galley, following the smell of breakfast and hot coffee, a peculiar sight caught my attention; lights in the distance. Deceived by a brain still half-asleep, it took me a minute to process what I saw. Since leaving Adak the last week the only signs of humans we’ve seen have been remnants of WWII and the flotsam that’s washed up on the beaches. Here, in one of the most remote places on the planet, we hardly expect to see another living soul. And yet, what looked like a small city twinkled persistently on the horizon. As the sky began to lighten I realized what we were seeing; the military base on Shemya. During the Cold War the US built a top-secret radar station here, and to this day the ominous “boom box” still listens for ballistic missiles, should they enter US airspace.  In transit on one of the inflatables, Dr. Edwards keeps a sharp lookout for the small floats that mark our sites In transit on one of the inflatables, Dr. Edwards keeps a sharp lookout for the small floats that mark our sites But we’re not here in the Semichis to gawk; we have our experiments to deploy and samples to collect. As stated before, the benthic productivity chambers (three in each of three habitat-types) are a beast to deploy. It takes all five divers from the Edwards Lab to set them up; we use lengths of heavy chain and whatever rocks we can find to make sure our chambers, and their respective sensors, stay upright all through the day and night. For those of you that might have missed it, inside each chamber we mount an oxygen and temperature sensor and a PAR sensor. PAR stands for “photosynthetically active radiation” or, more simply, the specific part of the light spectrum that plants and algae use for photosynthesis. The productivity chambers allow us to gain an understanding of what’s going in a fixed volume of water, which is important when we go to calculate the overall productivity of a system. What do we mean by productivity? We’re essentially looking at the difference between production (measured in the amount of oxygen produced) and respiration (the amount of oxygen consumed). Plants (and algae of course) and animals respire, but only photosynthesizers produce oxygen. Once we have a measurement of oxygen produced/consumed inside the chambers, we can compare that to the data gathered by the sensors we leave outside of the chambers, which are recording what’s going on in the environment. At the end of the 24-hr deployment, we “ground-truth” our measurements by collecting all of the organisms inside of the chambers when we go to pick them up and measure their biomass aboard the Oceanus. All the while we’re at sea, diving and steaming between islands, a colleague from Kunsan National University in Korea, Dr. Ju-Hyoung, and another Edwards Lab member, Sadie Small, run on-board experiments so we can get measurements on individual species’ rates of oxygen consumption/production.
Alright, I think that’s enough ecology for today. Be sure to check back in, after the Semichis we’re heading east again towards yet another island. Until then, this is Pike (aka Baron von Urchin) checking in from the Aleutian Archipelago |






















































 RSS Feed
RSS Feed
